
Clinical Trial Curation
Building a simpler, more streamlined curating experience to power clinical trial matching mechanism and feasibility study
Overview
Clinical trials are the primary ways medical researchers investigate if a new treatment (a drug or a therapy) - is safe and effective for people to use. While a single trial can be costly, patient recruitment takes up over 25% of the funds allocated to research trials. Genospace specializes in speeding up clinical trial research. With the extensive knowledgebase and advanced algorithms, we are able to match fragmented patient data to complex trial criteria and expedite patient recruitment. In order to successfully match patients to trials so that they can get the best-fit treatment, one critical component is the accurate capture of trial enrolling criteria, patient attributes and disease associations for a therapy drug.
Clarify ® by Genospace is an extensive application for precise medical information capture, but it started to face limitations as our trial database scaled. How could we improve the curation efficiency to speed up patient recruitment? I led the redesign process to improve Clarify usability and to extend its feature set.
My Role
I was the sole designer leading the Clarify Improvement Initiative.
My effort included leading the user research, concept development and UI design iterations.
I also worked with our clinical product owner to prioritize design deliverables into release candidates.
Tools & Methods
Jobs-to-be-Done Framework
Sketch & Invision
Problem
How to speed up trial curating efficiency while maintaining accuracy?

The lifecycle of a clinical trial matching mechanism starts with importing trial protocols into the system based on established medical oncologies. Genospace and Sarah Cannon curation teams use Clarify to establish and manage a central knowledgebase of trials and precision medical information. More specifically, the curation team is responsible to convert the protocol language (including amendments) into machine-readable inclusion/exclusion criteria with different weights. The standardized information will then be processed by the backend system and be used to match patients to the most suitable trials. As more trial protocols get entered into Genospace, along with constant amendments being added to protocols, curation team face challenges curating trials, and monitoring and validating changes quickly.
There are also limitations with the rather outdated interface which slow the curation efficiency. For example, the old Clarify layout doesn't enable recognition of output effectively. The rule creation process was also rather tedious, requiring many clicks, therefore slowing down curating efficiency. Certain functions (eg. version control) are missing, which make QA process more time-consuming.
At the same year, one of Genospace's business goals is to improve trial match specificity by 20%, which naturally requires more accurate trial curation. It was the perfect timing to start the Clarify Improvement initiative. (add some pics to demonstrate the current state)
Solution
A simpler, more streamlined curating experience by establishing version control, upgrading the criteria building interface, and incorporating trial feasibility evaluation.
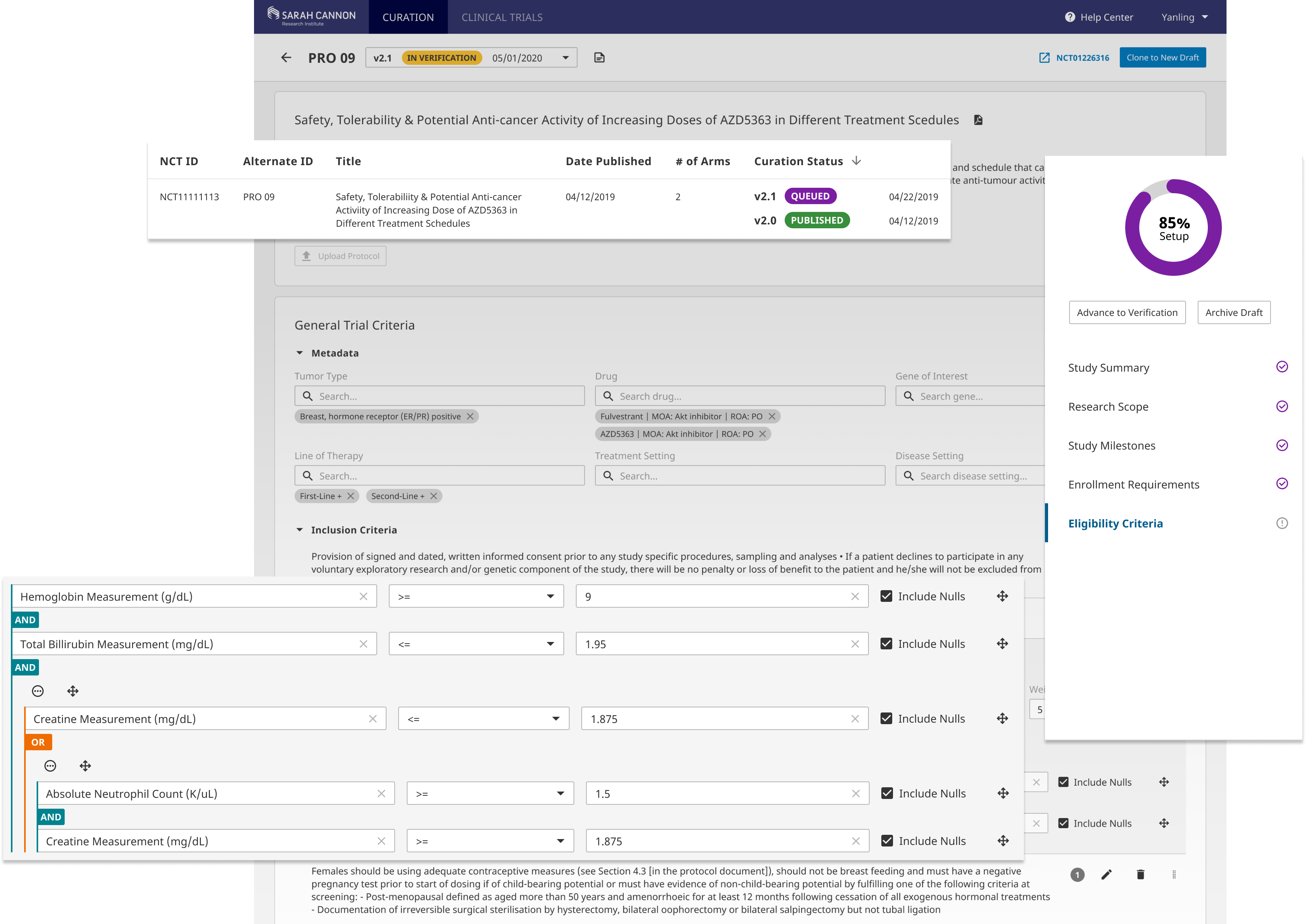
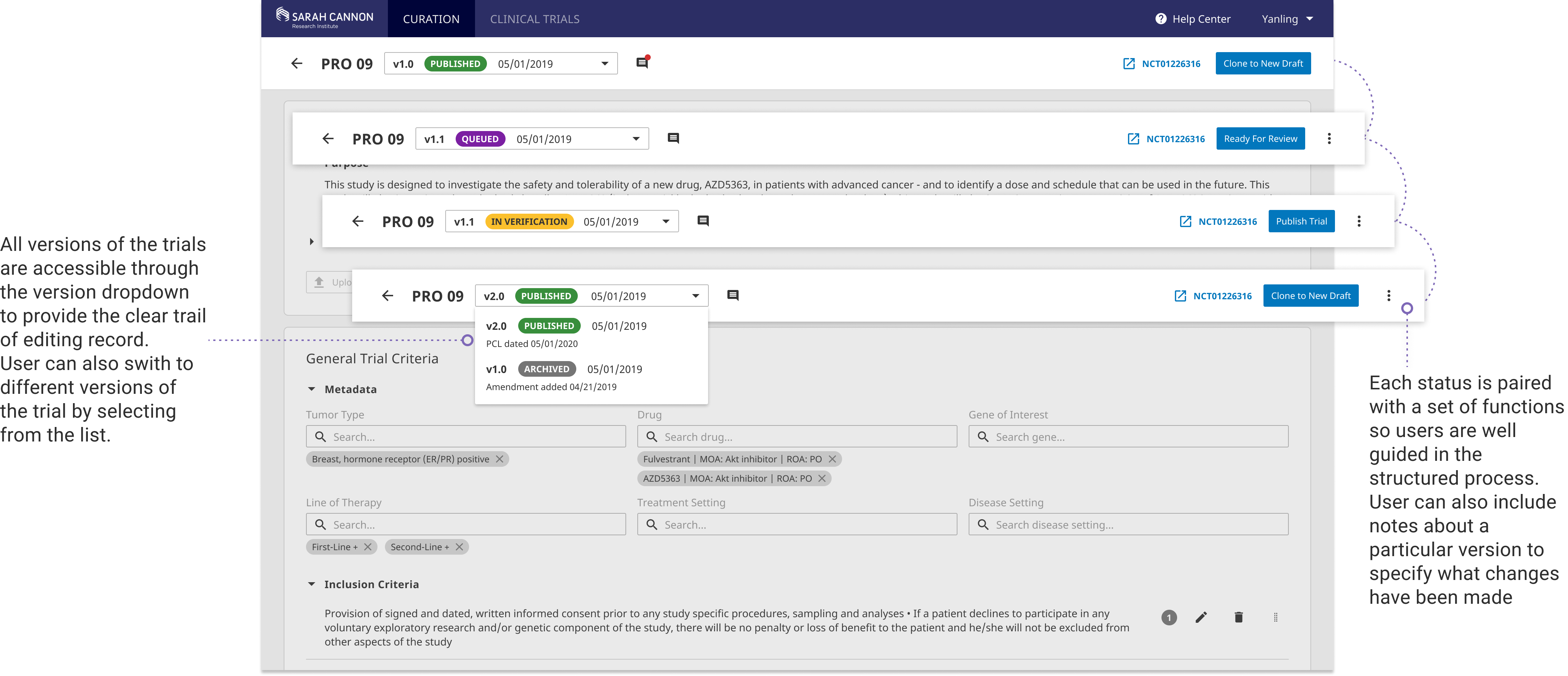
Curation Versioning Control
The main goal of curation versioning is to enable the curation of clinical trial amendments without disrupting the published versions of the trial so that the curation team can easily track changes to the trial protocol and maintain an audit trail with each published version.
By defining 4 trial curation statuses "Queued, Verification, Published, Archived", we encouraged a more standardized curation workflow. Each status is paired with a set of functions so users are well guided in the structured process.
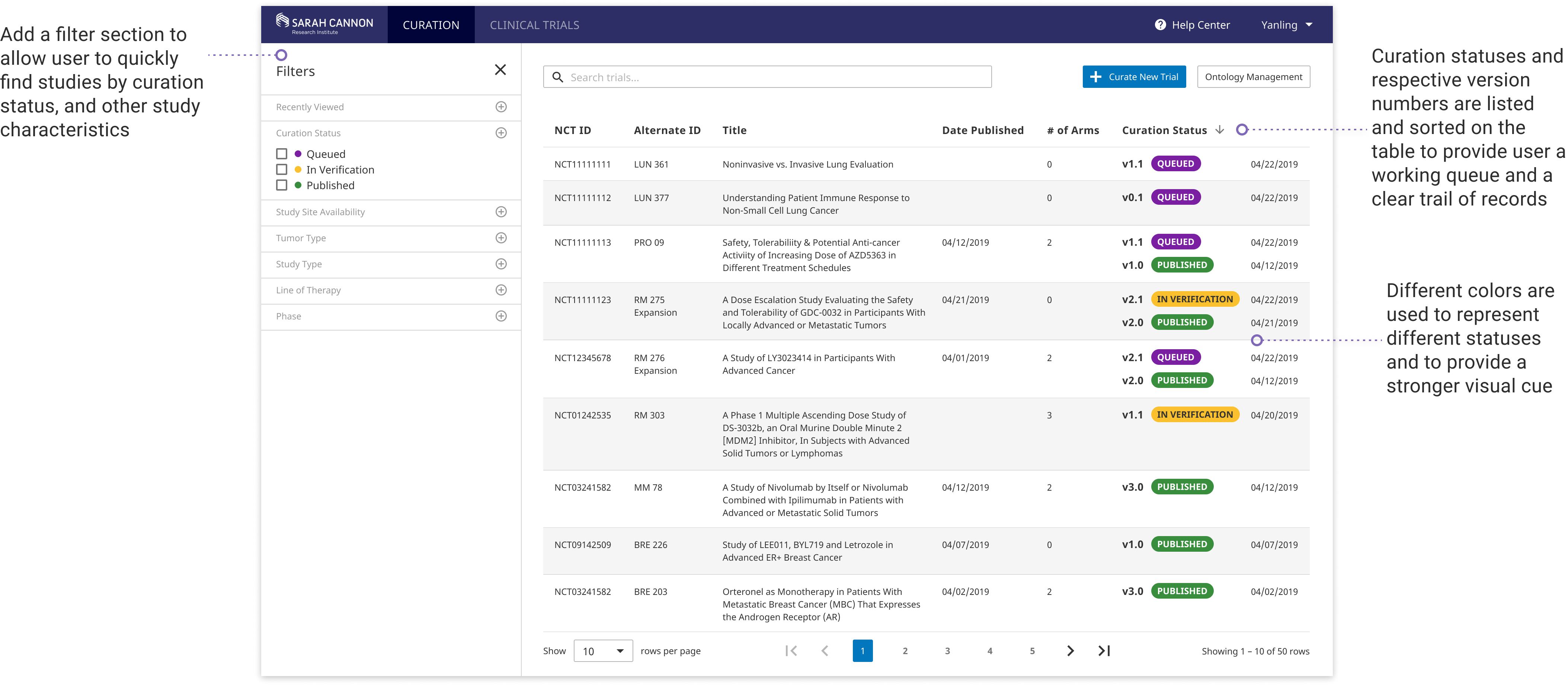
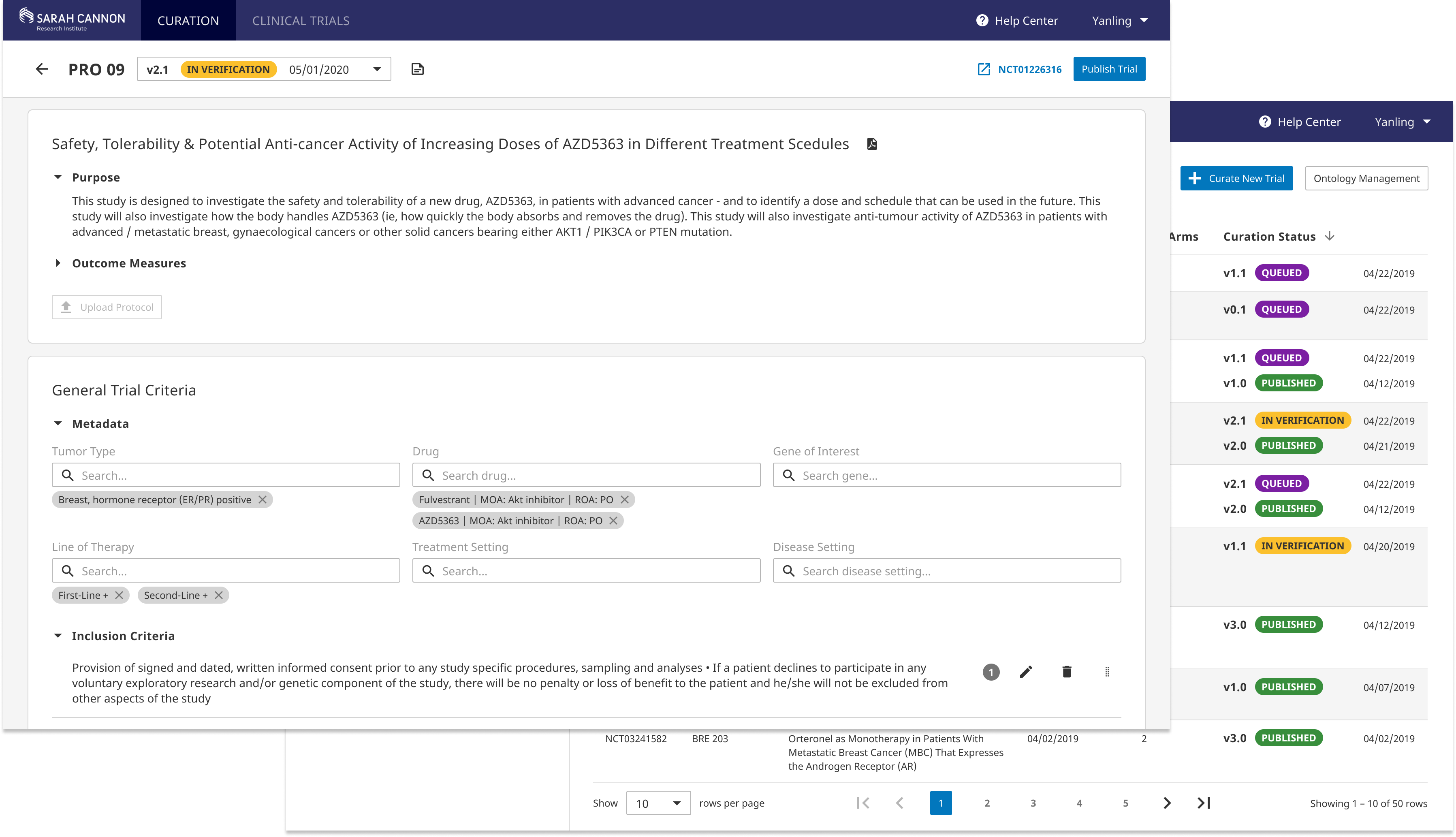
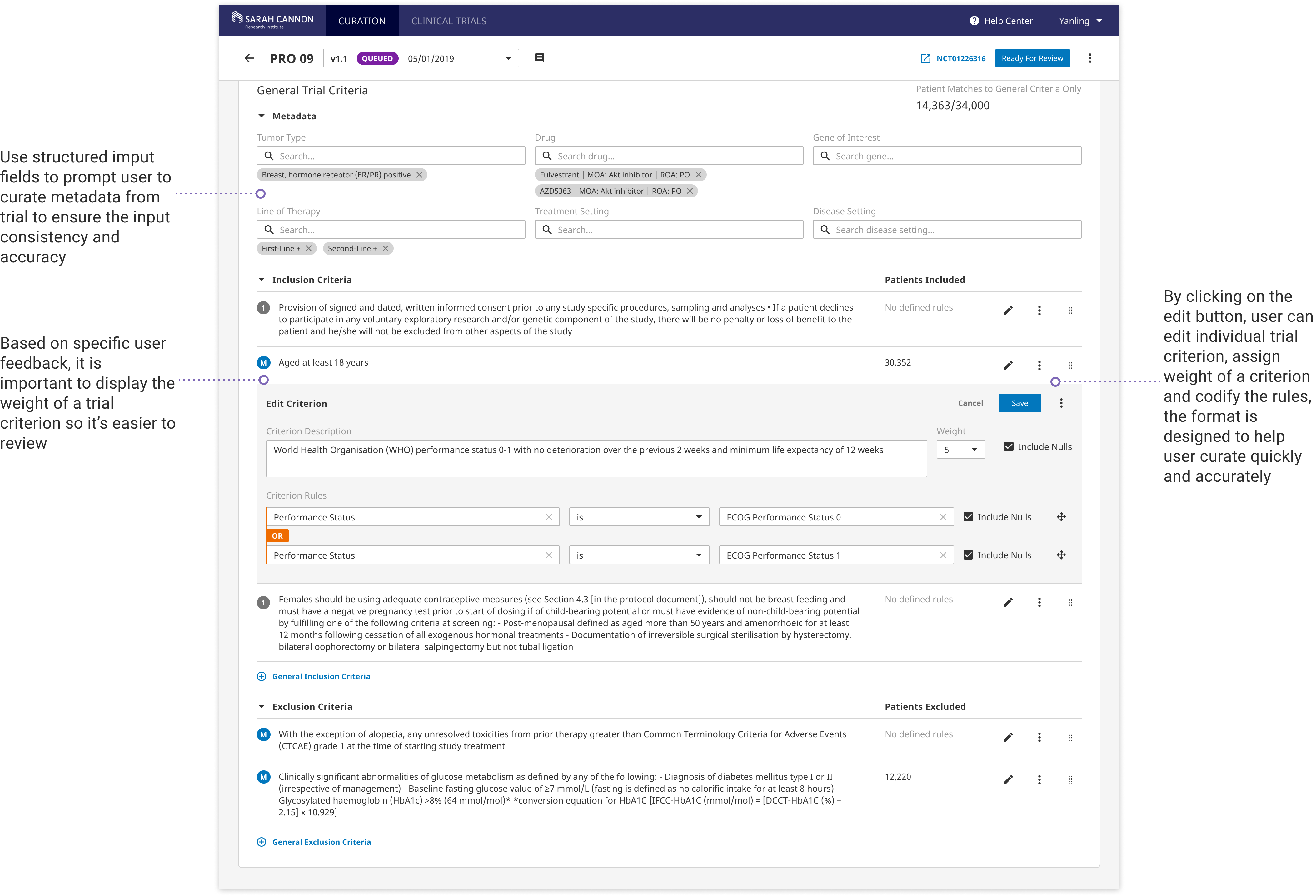
Powerful Curating Interface
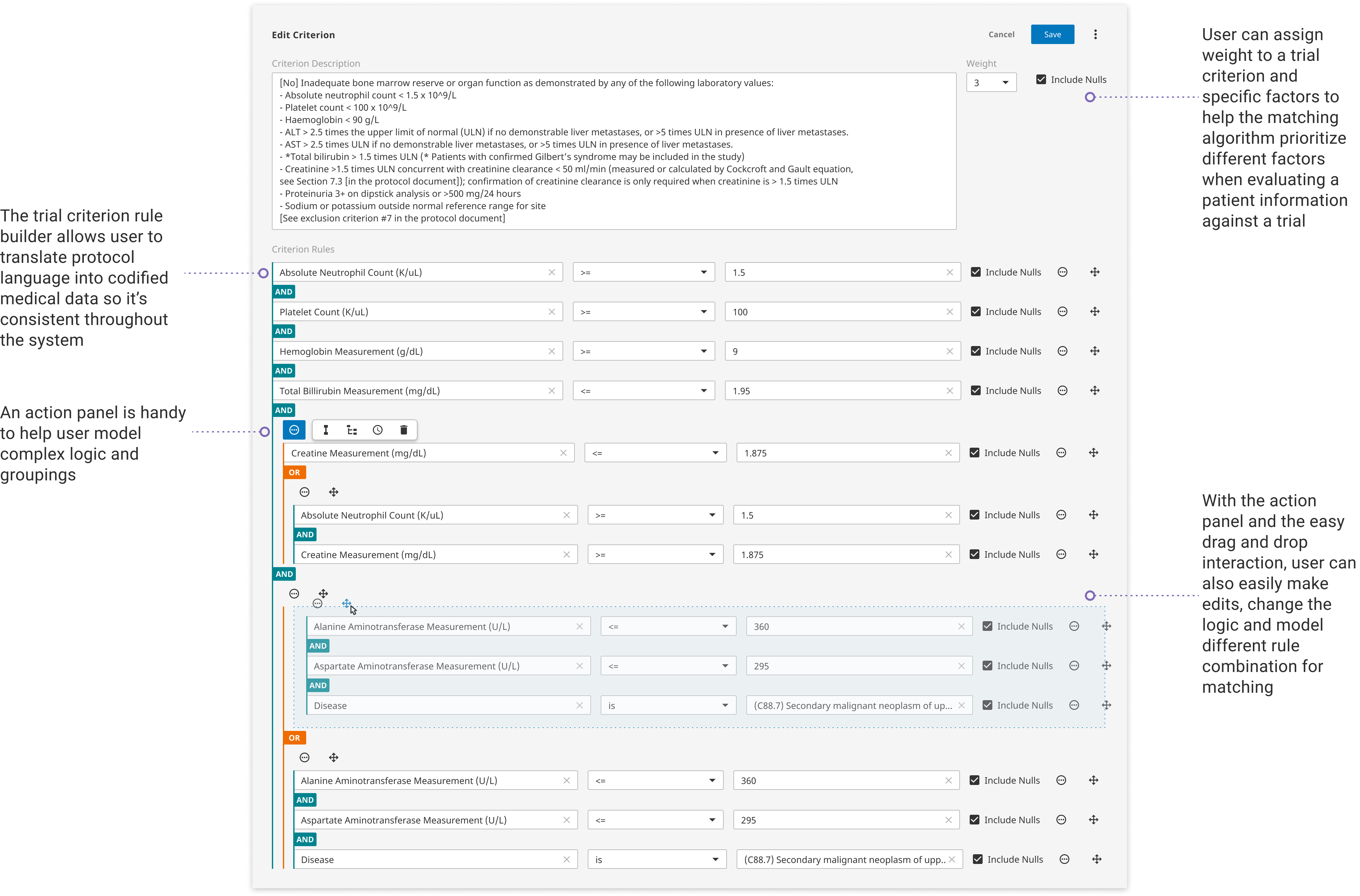
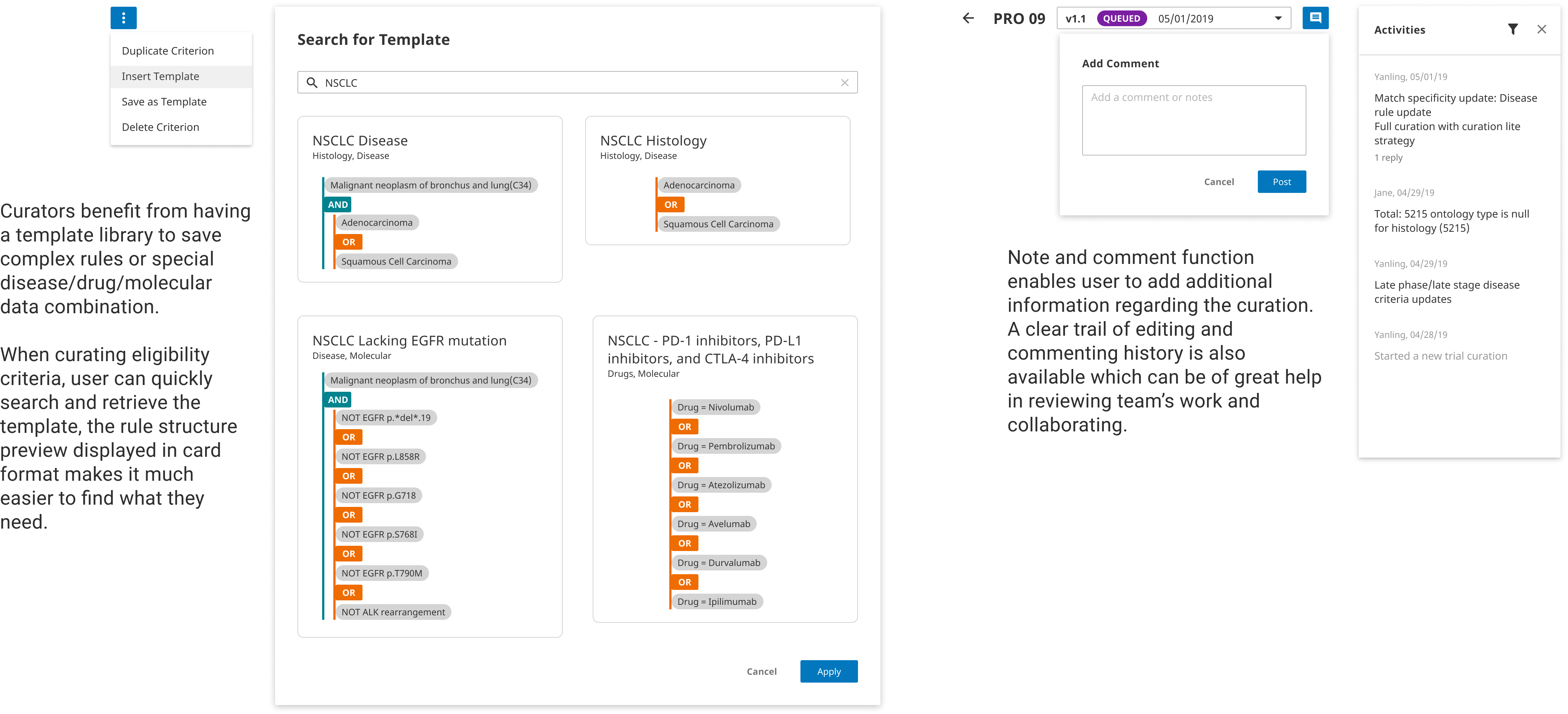
With the new full-width editing layout and structured input fields, users can quickly and accurately transform trial language into standard data input. Extensive utlities are enabled for creating complex rules, duplicating critera, and moving around fields. Reusable templates are included to help imporve curation efficiency.
Smart Trial Feasibility Evaluation
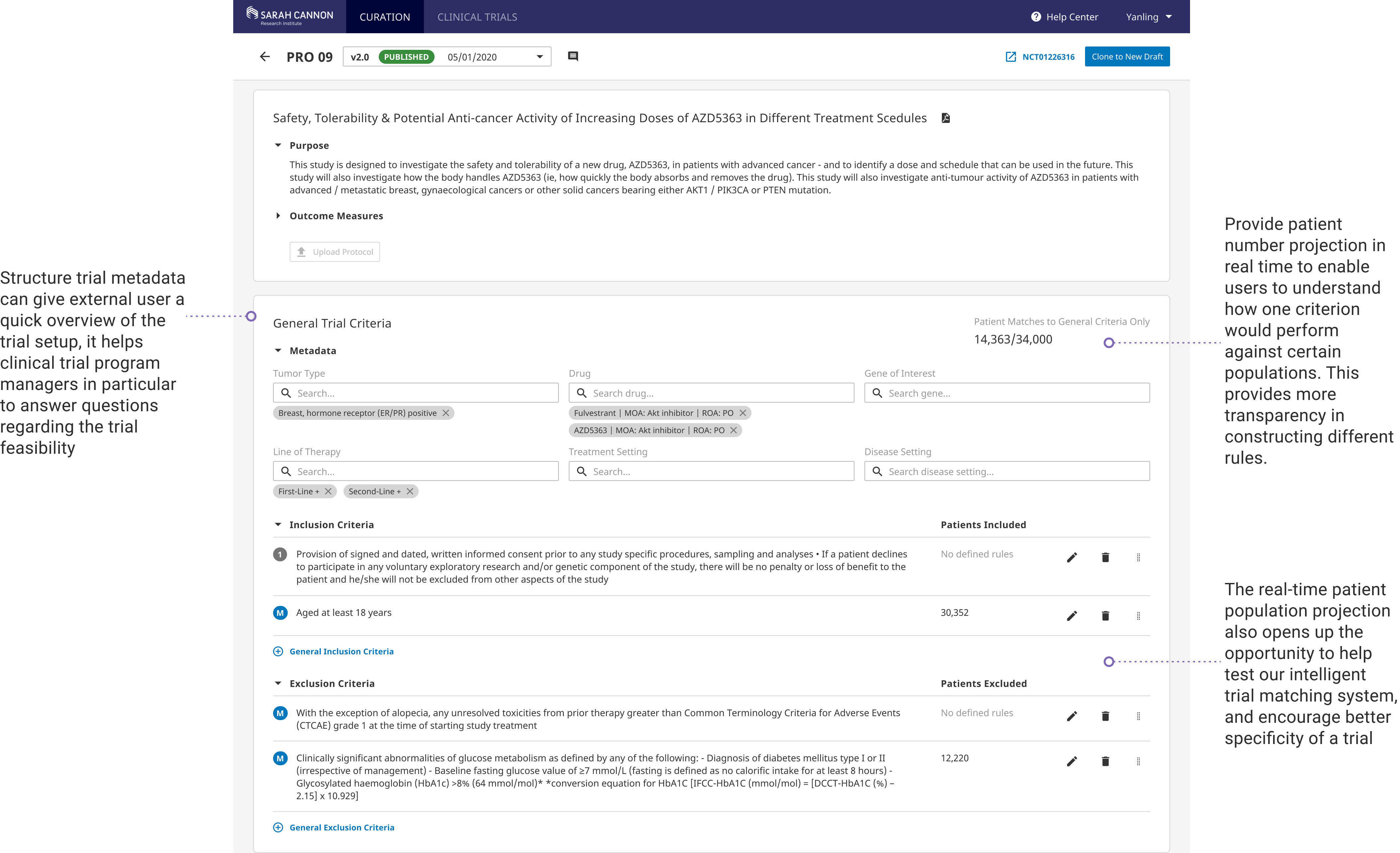
Trial feasibility projection is provided as user curates the trial to help curation scientists identify anomaly early in the trial development process. It also aims to engage clinical program managers to help them answer the feasibility questionnaire and to make more informed and strategic decisions when evauating trials.
Research
How did we arrive at the solution?
The Exploratory Research Process
As my very first product overhaul initiative at Genospace, I started off by interviewing with every curation team member to drive the problem-solving process. I conducted 4 individual contextual inqury interviews, faciliated 1 group disucssion. During my research process, I adopted the Job-to-be-done framework to help understand the curation workflow and uncover pain points and opportunities.
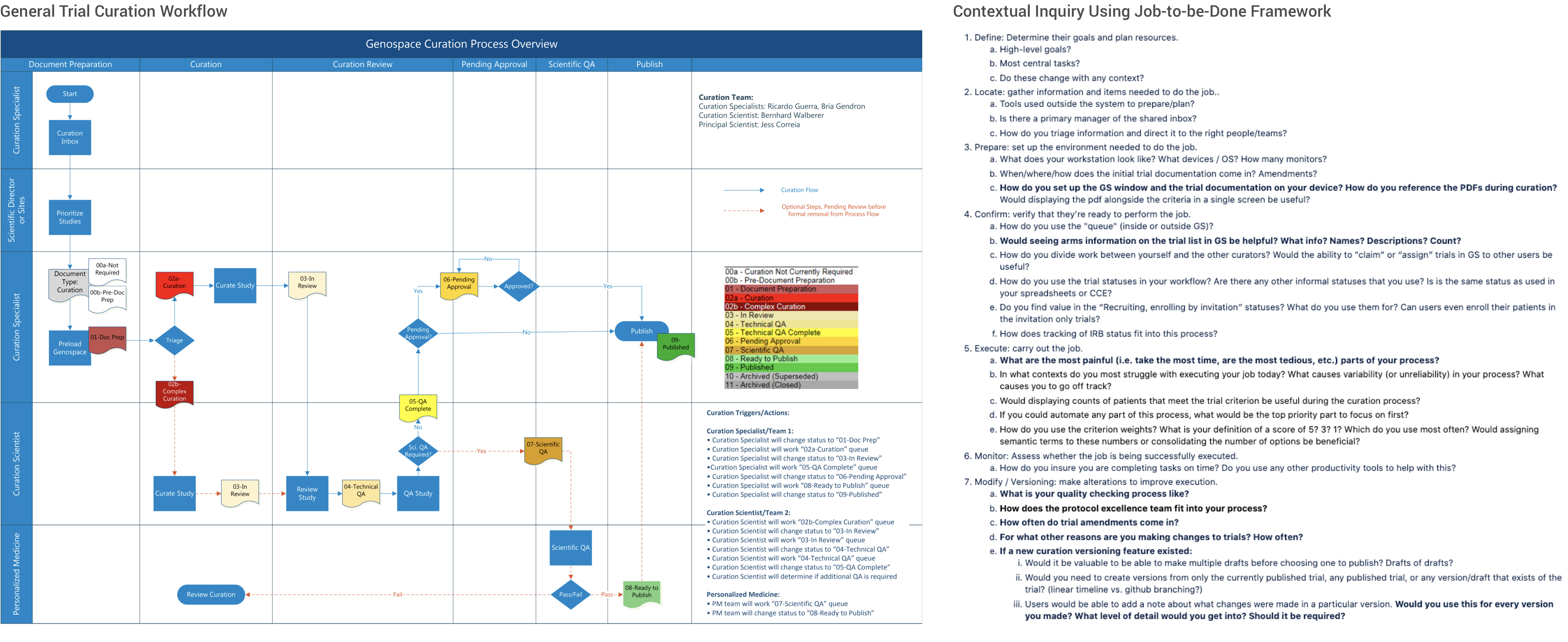
The Users and Things We Learned from Them
After the initial round of interviews with the curation team and evaluation of the application current state. I generalized the key user goals and opportunities for product improvement.
Who are we designing for?


While curation scientists are the main power users of Clarify, clinical research program managers can also benefit from Clarify to finish trial feasibility assessment. However, there are some major pain points that block curation scientists from curating more efficiently, and a lack of certain feature for program managers to really adopt the tool:

Version Control
The curation team was tracking progress and status outside of the system with a spreadsheet. As study amendments come in, the team had to use IM to constantly communicate changes so they don't override each other's work. Therefore, enabling curation changes without disrupting the published work is a critical user requirement.
Curation Interface Usability
The current curation interface to work with is rather outdated. The trial criteria layout doesn't enable recognition of weight assignment or navigation effectively. Certain rule building can get extremely complicated and difficult to review.

Template Saving & Management
The curation team use various forms of templates to speed up the criteria building process and to avoid errors. However the current template library has great limitation in funcationality, it has been more difficult to find a useful template than building one from scratch.

Trial Feasibility Component
The end goal of curating a trial protocol is essentially matching patients to personalized treatments. Adding the trial feasibility feature in curation is a natural step to help program managers and curation scientists understand if a trial is worth pursuing.
The Strategy
Co-designing with Users & Prioritize Solutions on an Effort/Impact Scale
While we identified that there are multiple areas of improvement that can be made for Clarify. It would be a big engineering lift to implement all features at the same time along with other company initiatives. Therefore, we broke down the features by feasibility, desirability, and viability. We also evaluated individual feature by the effort/impact scale to decide on which feature to develop first that can yield largest impact for users. (add a pic of feasibility evaluation)
After careful evaluation with the product and the engineer teams, we decided to implement the Clarify Improvement Initiative in 3 releases:
- Phase 1: Enable Clarify Versioning Control
- Phase 2: Clarify Usability Fix & Interface Upgrade
- Phase 3: Clarify Feature Expansion - Trial Feasibility
Since this product is mainly used by a small team of curation scientists, I tried a new way of working with users, bringing them into every stage of the design process. By meeting with the curation team regularly to review design and get concrete design feedback, together we prototyped, built and refined the designs to create a simpler and a more streamlined trial curating experience.
Design
Iterate, iterate, iterate
The Clarify redesign process is a rather complicated process that involves much detailed workflow mapping and interaction design. Throughout the concept development process, I benefited much from constantly reviewing the design with my user panel - the curation team and prototyping with real data and concrete examples.
It helps me take the complexity into consideration from the very beginning. Among all the different aspects of the design iteration, below are some examples of how my designs evolved along the way:
The Layout
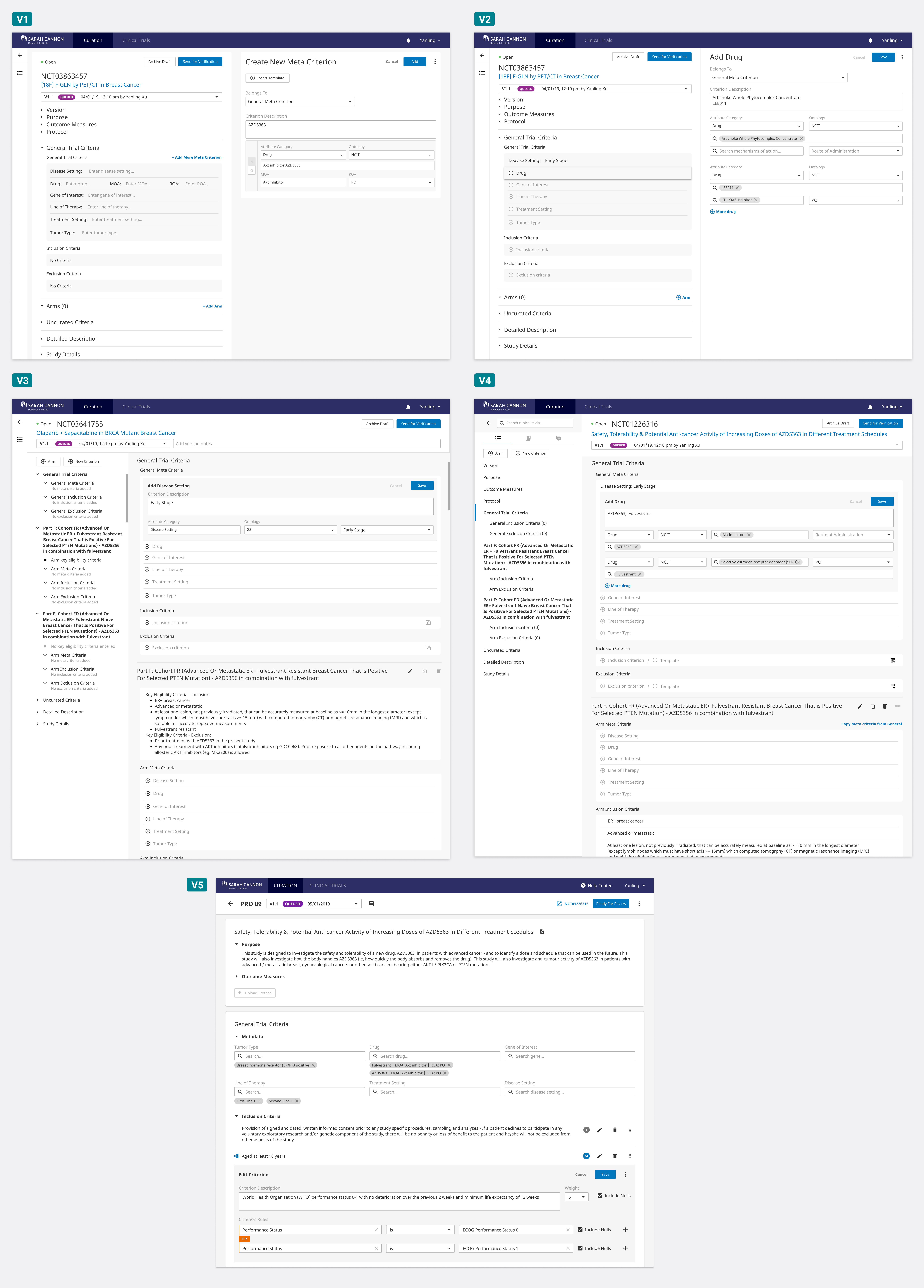
The original layout of the Clarify curating interface was a 50/50 form where user can scroll the left side content and fill out the form on the right. While it's useful to have the curation and the editor side by side, the original layout wasted a lot of space and requires a lot of scrolling.
My initial exploration around better curation UI was rather conservative. I wanted to create a design that needs minimal engineering effort. But as I spoke with users and the engineering team, it turned out that the layout change wouldn't be a huge lift. Users can also benefit a lot from having more space when editing trial criteria and rules. I then started to pursue a full-width layout and explore how to best navigate around it.
The Rule Builder Exploration
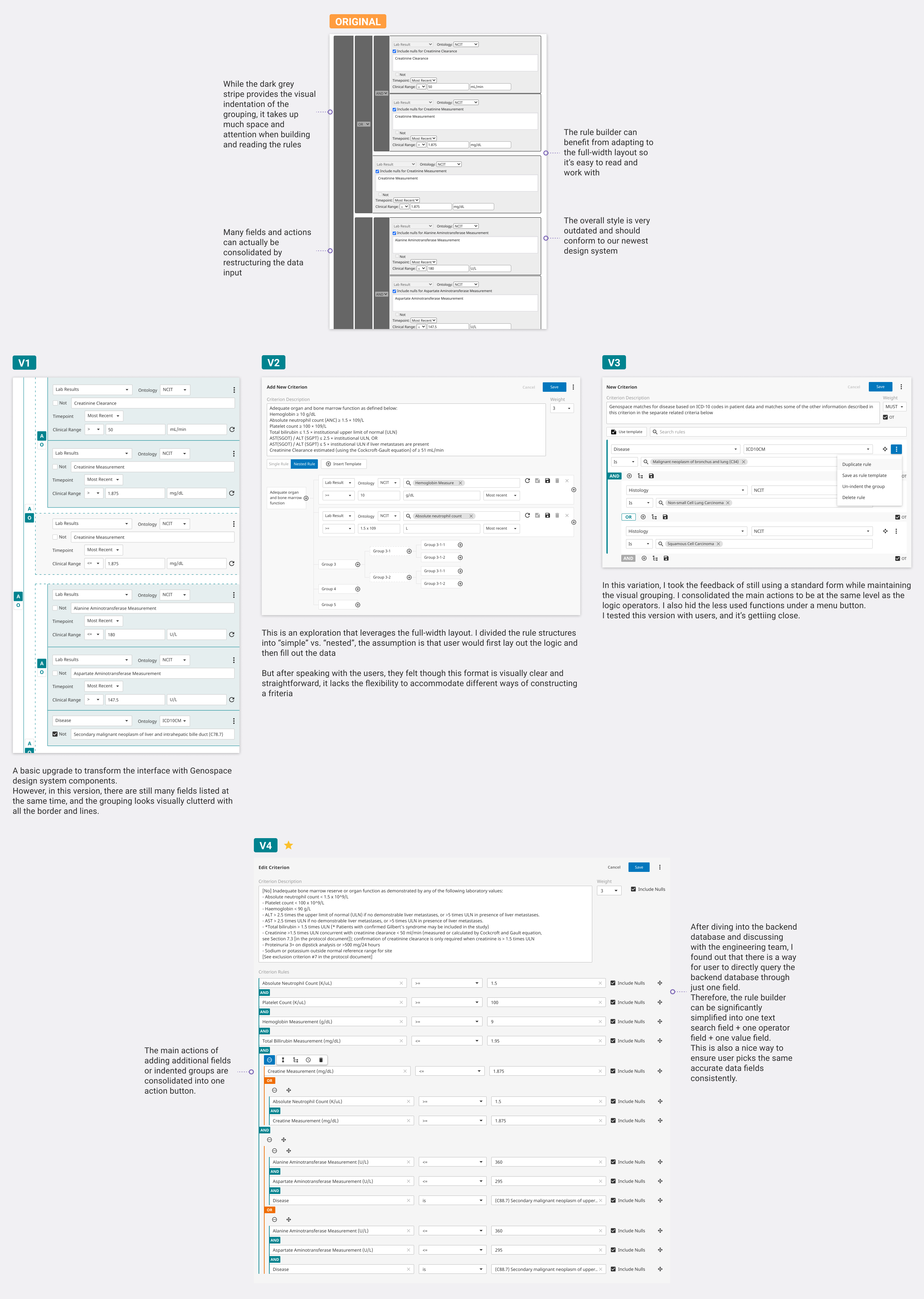
The rule builder is one of the most chanllenging part in developing the new UI for Clarify because the rule builder is essentially a combiination of a form and a query builder that translates plain langugae into system-recognizable data. On the one hand, user needs to be able to quickly transform text into structured data, on the other hand, user also needs the flexibility to construct complex logic that feeds into the matching algorithm.
More specifically, user needs to be able to map data onto different established medical categories. User also needs to create different condition groups using the rule builder. The original rule builder was quite comprehensive, but it lacked visual clarity and can be extremely hard to read when a rule gets very complicated. I spent a lot of time exploring different styles and components to construct a powerful yet elegant rule builder. I also gained user feedback not only from curation team but also general users to make sure the interaction is truly easy to use.
As a bonus result of the exploration, the rule builder concept was adopted by other team members in other products.
Outcome
This was a really exciting and fun initiative for me to work on as it was one of my first full-scope product initative, it provides real business and user value, involved a lot of research, and detailed design work. As mentioned above, the whole Clarify improvement was broken up into several relase candidates, and the curation versioning workflow and the UI improvement have been implemented and launched as new features. Throughout the process, I learned a tremendous amount related to product design and development with users.
How to design in a new domain
This will always be a challenge when designing for enterprise products and professional users. I benefited significantly from having a user panel right at the beginning of the project. It's also important to establish a good relationship with my user panel to constantly listen to them and absorb feedback. It's also the best way to familiarize myself of a new domain.
How to prioritize and utiliize a large solution concept
While it's always preferrable to come up with the full-scope, end-to-end solution based on the user journey. It is important to understand how to break down large concept into chunks and prioritize features when coming to actual implementation. I learned a lot by working with the product owner and the engineering lead to understand how best evaluate a new feature and the implementation effort.
Always fight for good UX
While I had to work with very strict technical constraints, I still fought for what I believe is essential to having a good user experience.
Thank you for reading this far : )

